Heterogeneous Catalysis: Interactions of Molecules with Surfaces
Heterogeneous catalysis is one of the key technologies in the chemical industry. Many economically important chemical processes are heterogeneously catalyzed. The most common heterogenoeus catalysts are metal-nanoparticles with porous oxide or carbon as support. Up to the present, the elementary steps and the parameters which determine the reactivity are unknown for many of such heterogeneous catalyzed reactions. For supported metal-nanoparticles the interactions between the support material and the nanoparticles has only for a few examples been studied satisfyingly. Furthermore, a lot of phenomena observed in experimental studies like the influence of surface defects and the hydrogen spillover mechanism cannot be explained so far.
Our group has a longtime experience with computational studies on the electronic structure of surface defects in the context of heterogeneous catalysis. Within the collaborative research center (SFB 558) we studied between 2006 and 2012 the reaction pathways for the methanol synthesis at oxygen vacancies at the polar 000-1 ZnO surface. We also investigated how the doping of the zinc oxide surfaces influences the activation of molecular hydrogen and the electronic properties of the catalyst and characterized adsorbates of N2 and NO on TiO2 (rutil) surfaces. Further details are given here.
Methanol Synthesis at an Oxygen Vacancy on the Polar ZnO(000-1) Surface
Formation of weakly bound, ordered adlayers of CO on rutile TiO2(110)
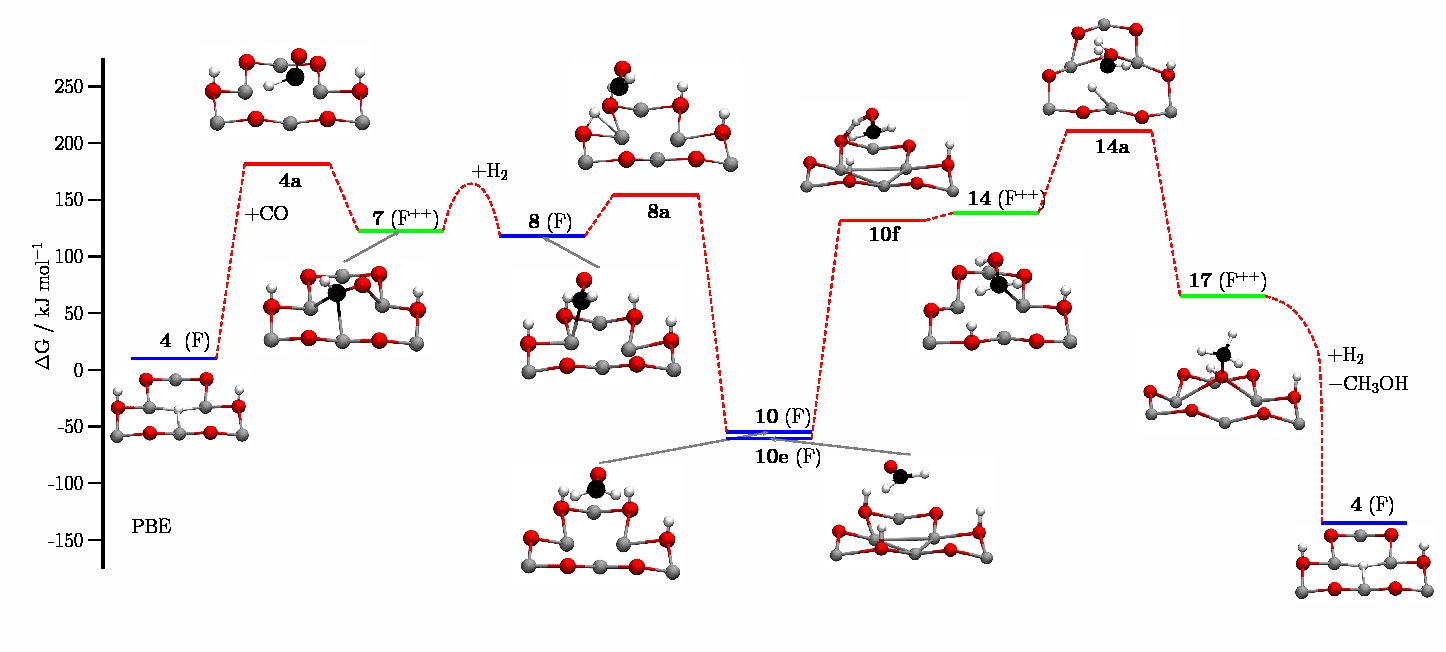
From 2017 to 2022, within the framework of the Sino-German initiative "Novel Functional Materials for Sustainable Chemistry" we studied hydrogenation reactions on carbon supported palladium clusters. The purpose of this project was to reveal the influence of support materials on the electronic properties of metal catalysts and their influence on the mechanisms and barriers of the catalyzed reactions. We wanted to obtain an in-depth insight into metal-support interactions and the importance of metal-support interactions for the whole process of heterogeneous catalysis. To study the complex interplay between hydrogen, metal-nanoparticles and support material, we performed state-of-the-art electronic structure calculations. One of our main interests was to study the influence of heteroatoms in the support material on the heterogeneous catalyzed hydrogenation reaction.
Anchoring of palladium nanoparticles on N-doped mesoporous carbon
A quantum chemical study of hydrogen adsorption on carbon-supported palladium clusters
 In the period from 2018 to 2022, our group was involved in the new CRC/Transregio 247
"Heterogeneous Oxidation Catalysis in the Liquid Phase – Mechanisms and Materials in Thermal, Electro-, and Photocatalysis"
with
project A5 "Quantum Chemical Investigation of Catalytic Cycles on Transition Metal Oxides".
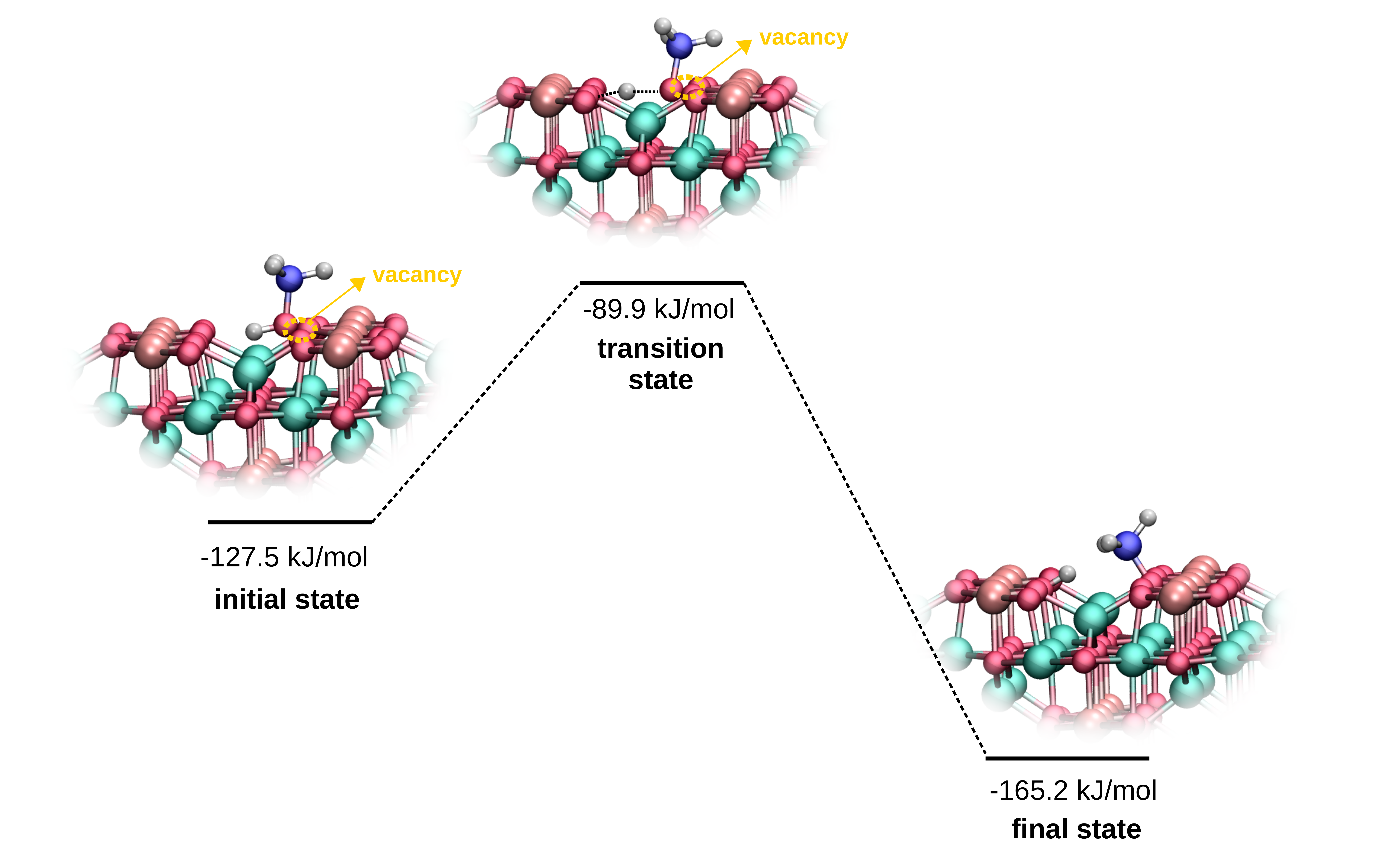
We focused on the aerobic selective oxidation of short-chain alcohols: methanol, ethanol, and 2-propanol, on the CoFe2O4 (001) surfaces, in the gas phase.
Special attention was placed on the formation of oxygen vacancies, due to their key role in the oxidation activity of metal oxides,
often based on the Mars-van-Krevelen mechanism.
We implemented the Periodic Electrostatic Embedded Cluster Model (PEECM)
within the TURBOMOLE software package for studies in the limit of low coverage.
To investigate and identify the rate limiting elementary steps of the catalytic reaction cycles and the most important intermediates,
we used the woelfling program at the DFT/PBE0 level of theory.
In the period from 2018 to 2022, our group was involved in the new CRC/Transregio 247
"Heterogeneous Oxidation Catalysis in the Liquid Phase – Mechanisms and Materials in Thermal, Electro-, and Photocatalysis"
with
project A5 "Quantum Chemical Investigation of Catalytic Cycles on Transition Metal Oxides".
We focused on the aerobic selective oxidation of short-chain alcohols: methanol, ethanol, and 2-propanol, on the CoFe2O4 (001) surfaces, in the gas phase.
Special attention was placed on the formation of oxygen vacancies, due to their key role in the oxidation activity of metal oxides,
often based on the Mars-van-Krevelen mechanism.
We implemented the Periodic Electrostatic Embedded Cluster Model (PEECM)
within the TURBOMOLE software package for studies in the limit of low coverage.
To investigate and identify the rate limiting elementary steps of the catalytic reaction cycles and the most important intermediates,
we used the woelfling program at the DFT/PBE0 level of theory.
Structure and Reactivity of Pristine and Reduced Spinel CoFe2O4 (001)/(100) Surfaces
Activation of Molecular O2 on CoFe2O4 (001) Surfaces: An Embedded Cluster Study
Interactions of Water and Short-Chain Alcohols with CoFe2O4 (001) Surfaces at Low Coverages
In May 2022, the DFG funded the second period of CRC/Transregio 247. Currently, we focus on the reaction sites and mechanisms at the solid/liquid interface, by combining PEECM with the conductor like screening model (COSMO) as a polarizable continuum solvation model. In addition, we compare the reaction mechanisms on periodic surfaces with those on molecular catalyst models. The ultimate goal is to develop, in cooperation with spectroscopy projects, an atomistic description of CoFe2O4 and related spinel surfaces, beyond the ideal bulk crystal structure and under conditions of catalytic reactions in the gas and liquid phase.